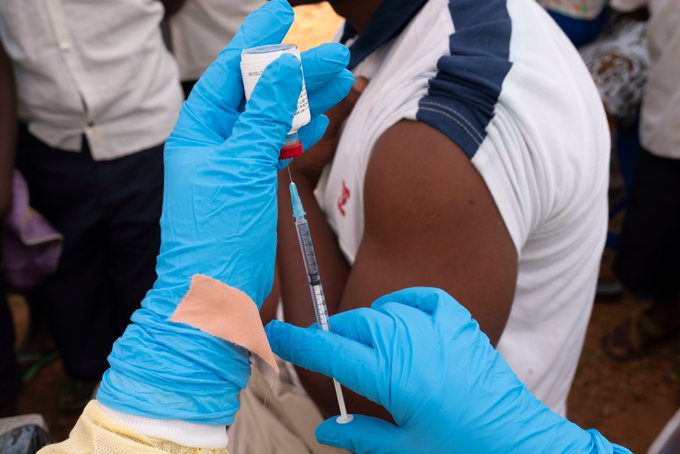
The World Health Organization (WHO) today prequalified an Ebola vaccine for the first time, a critical step that will help speed up its licensing, access and roll-out in countries most at risk of Ebola outbreaks. This is the fastest vaccine prequalification process ever conducted by WHO.
Prequalification means that the vaccine meets WHO standards for quality, safety and efficacy. United Nations agencies and Gavi, the Vaccine Alliance, can procure the vaccine for at-risk countries based on this WHO recommendation.
“This is a historic step towards ensuring the people who most need it are able to access this life-saving vaccine,” said Dr Tedros Adhanom Ghebreyesus, WHO Director-General. “Five years ago, we had no vaccine and no therapeutics for Ebola. With a prequalified vaccine and experimental therapeutics, Ebola is now preventable and treatable.”
The injectable Ebola vaccine, Ervebo, is manufactured by Merck (known as MSD outside the US and Canada). It has been shown to be effective in protecting people from the Ebola Zaire virus and is recommended by the WHO Strategic Advisory Group of Experts (SAGE) for vaccines as part of a broader set of Ebola response tools. The decision is a step towards greater availability of the vaccine in the future, though licensed doses will only be available mid-2020.
This announcement comes less than 48 hours after the European Commission decision to grant a conditional marketing authorization for the vaccine, following the recommendation from the European Medicines Agency (EMA).
Due to the urgent public health need for a prequalified Ebola vaccine, WHO accelerated prequalification by reviewing safety and efficacy data as the information became available. Representatives from the prequalification team participated in the EMA evaluation process to address programmatic suitability for at-risk countries in Africa.
“The development, study, and rapid prequalification of this vaccine show what the global community can do when we prioritize the health needs of vulnerable people,” said Dr Tedros.
WHO is also facilitating licensing of the vaccine for use in countries at risk of Ebola outbreaks, based on the reviews and positive outcome by the EMA. WHO, with the support of EMA, has worked closely with many African regulators who have indicated they will quickly license the vaccine following the WHO recommendation.